Verrutop is an effective topical solution for Warts and Verrucae

A new therapeutic treatment for palmoplantar and periungual warts, with Nitrizinc Complex®.
Verrutop® doesn't freeze or burn tissues like cryotherapy or other chemical treatments.
By denaturing the viral protein, it dries out the wart tissue and causes it to fall off the skin, leaving intact skin underneath.
Verrutop®is a Class IIa Medical Device. Verrutop® is not intended for home use by members of the public.
High efficacy in plantar, palmar and periungual warts.
What is Verrutop®?
Verrutop® is formulated with Nitrizinc Complex®, a solution containing nitric acid, organic acids, zinc and copper salts.

How does it work?
Verrutop® acts by denaturating the wart proteins through a caustic effect (mummification).
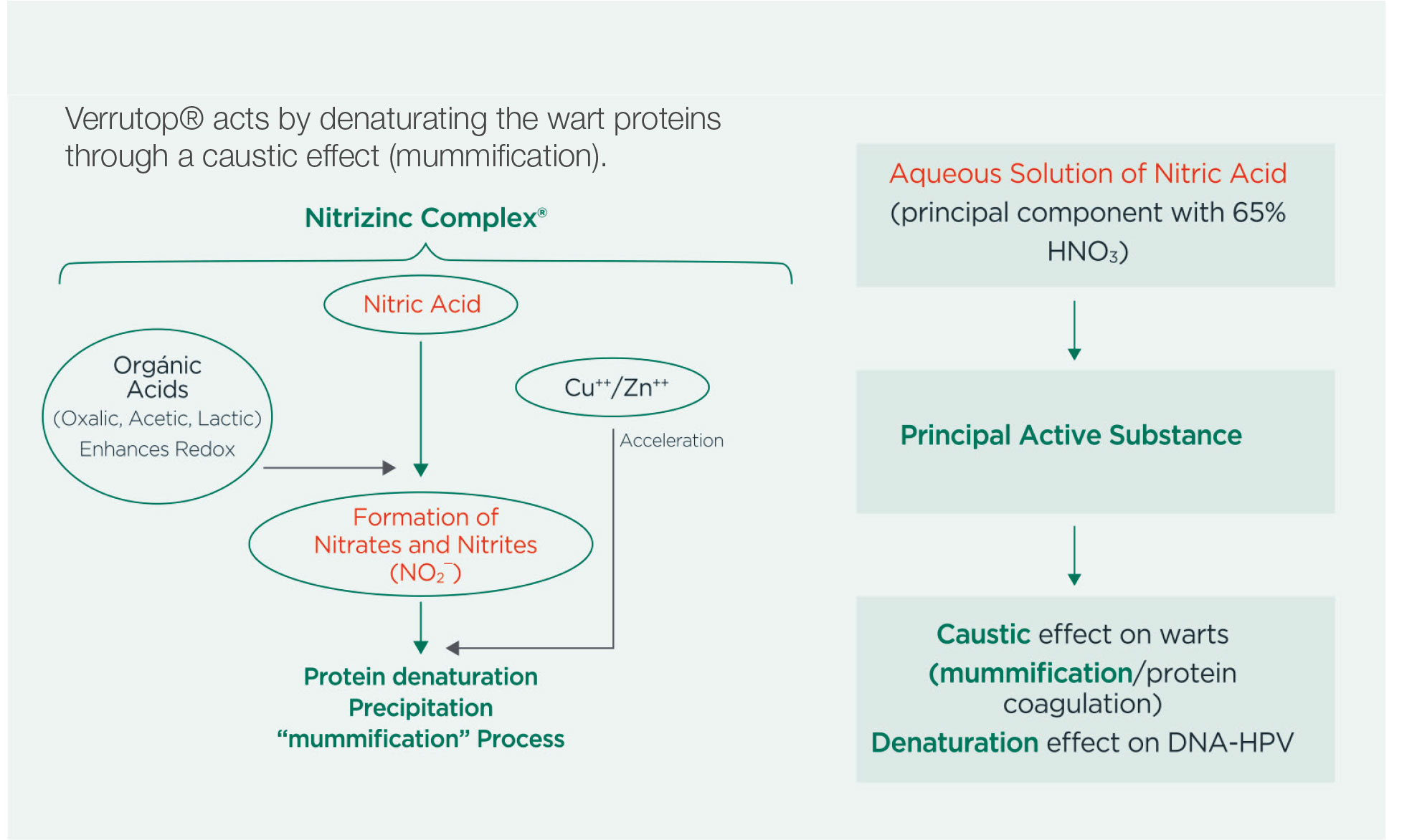
- Verrutop® causes a redox reaction and generates nitrites (N02-), by denaturing viral proteins.
- Necrosis (death of cells) is initiated by the nitric acid and catalyzed by the production of nitrite.
- Protein degradation induced by N02- is accelerated by the metallic salts contained in the Verrutop® formulation.
Verrutop® is quick and easy to apply.
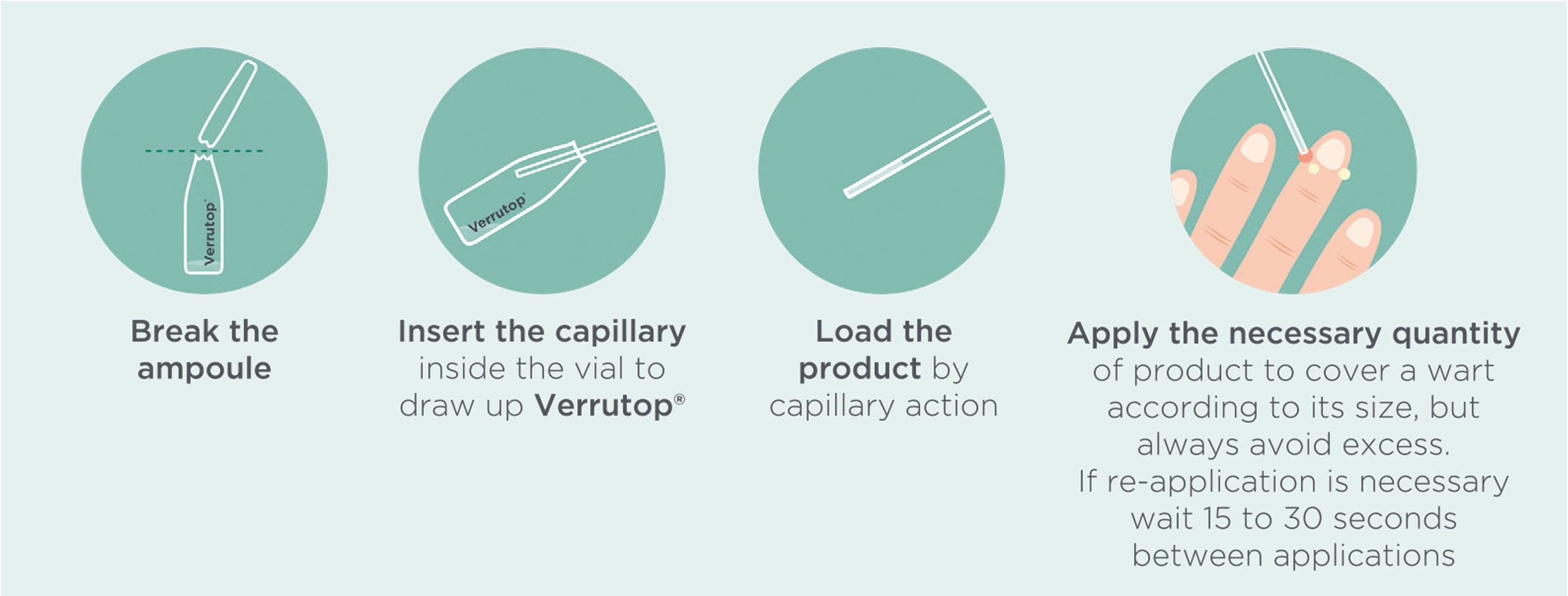
Helpful tips:
- On highly keratinized warts it is advisable to eliminate the thickened surface layer using a scalpel or similar instrument in order to improve the penetration of Verrutop®.
- Ask the patient to apply surgical spirit twice a day to the wart to assist in the dehydration process.
- Assess the patient 1-2 weeks after the treatment and reapply if necessary.
Do not use Verrutop®:
X On healthy skin, on inflamed skin or mucous membranes, on malignant cutaneous tumours, on flat warts, on
ephelides (freckles) or on keloids (intense scarring reaction)
X On children under the age of 6 years
X On women who are pregnant or breast feeding
X On patients with arteriopathy and/or peripheral neuropathies such as seen in some diabetics. In case of a concomitant
cutaneous pathology a doctor will decide on the risk/benefit of treatment
X As concomitant treatment with other topical wart removers.
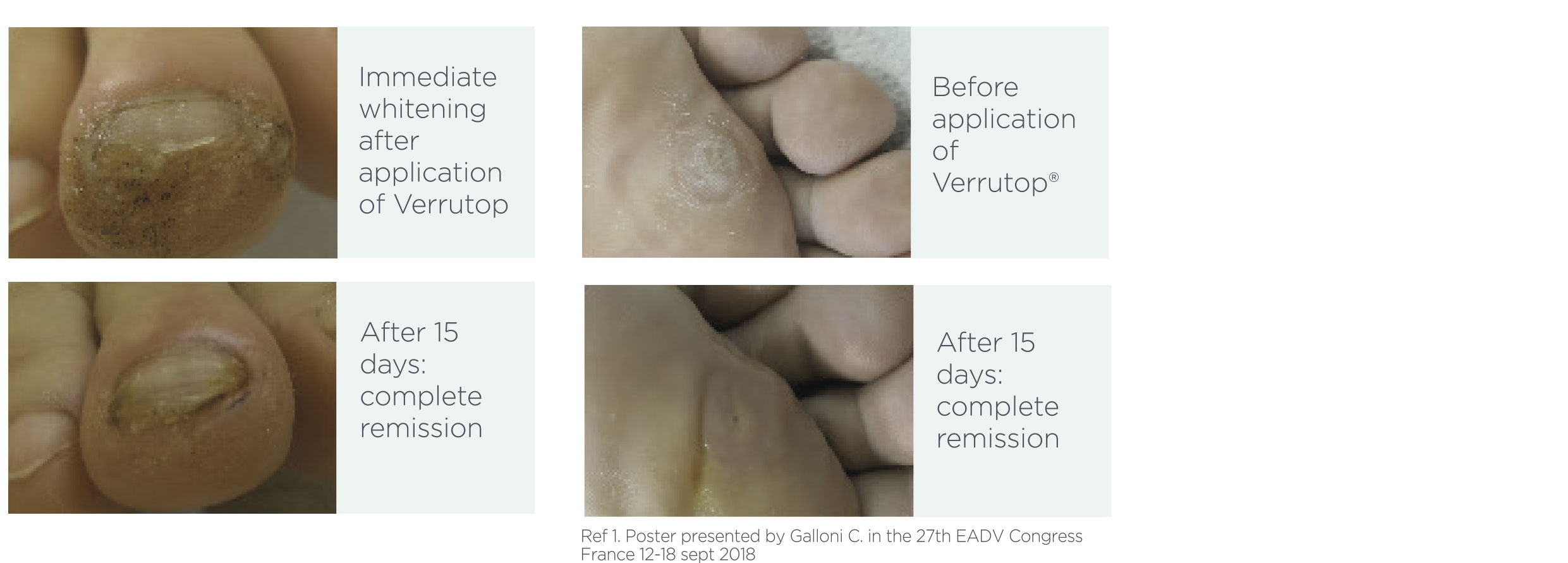
Verrutop® has shown high efficacy and tolerability in palmar plantar and periungual warts.
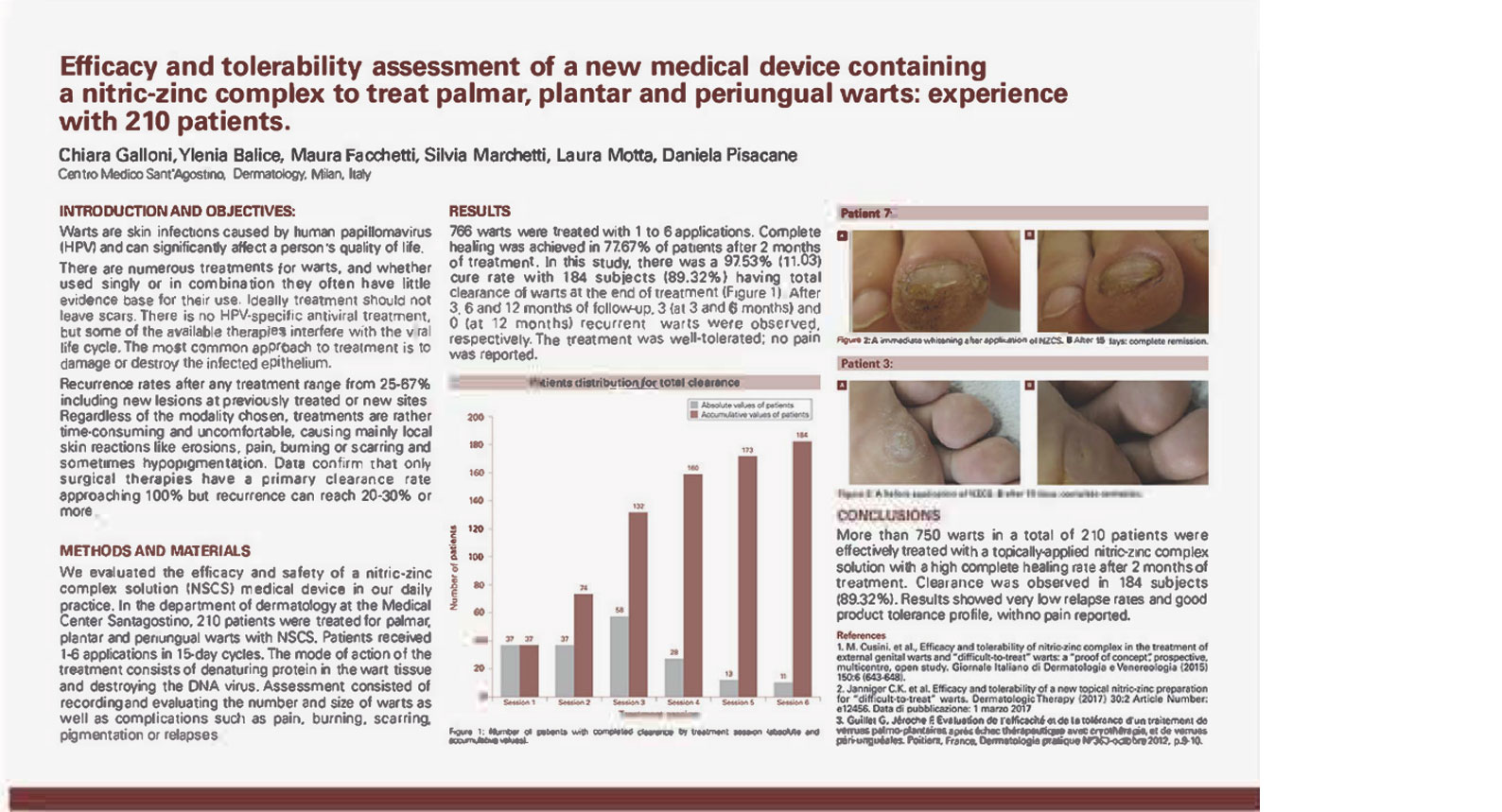
Efficacy and tolerability assessment of a new medical device containing a Nitrizinc Complex® to treat palmar, plantar and periungual warts: experience with 210 patients. 766 warts treated.
• Complete remission in 89% of patients with a maximum of 6 topical applications of Verrutop®
• More than 50% of patients showed complete clearance of warts treated with 3 sessions of Verrutop®
• Results showed very low relapse rates (3 cases at 3 and 6 months and 0 cases at 12 months) and good product tolerance, with no pain reported.
Ref 1. Poster presented by Galloni C. at the 27th EADV Congress France 12-18 sept 2018 N= 766 warts.

Ref2
Assessment of the efficacy of a new formulation for plantar wart mummification: new experimental design and human papillomavirus identification.

With Verrutop®, the complete viral HPV DNA elimination occurs in 40% of cases while no effect was achieved by liquid nitrogen2.
1. Galloni C. et al. Efficacy and tolerability assessment of a new medical device containing a nitri-zinc complex to treat palmar, plantar and periungual warts: experience with 201 patients. 27 th EADV congress. France 12-18 sept 2018. 2. Viennet C et al. Assessment of the efficacy of a new formulation for plantar wart mummification: new experimental design and human papillomavirus identification.
CED a 2012 British Association of Dermatologists Clinical and Experimental Dermatology, 38, 8S-88

